Just For You More
| id | 1923218244785188866 |
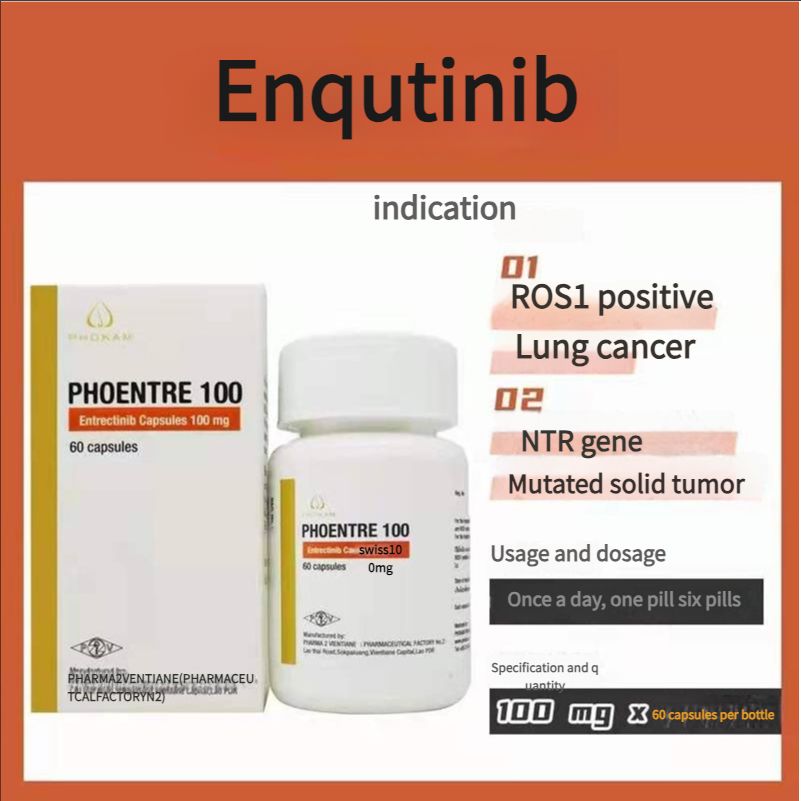
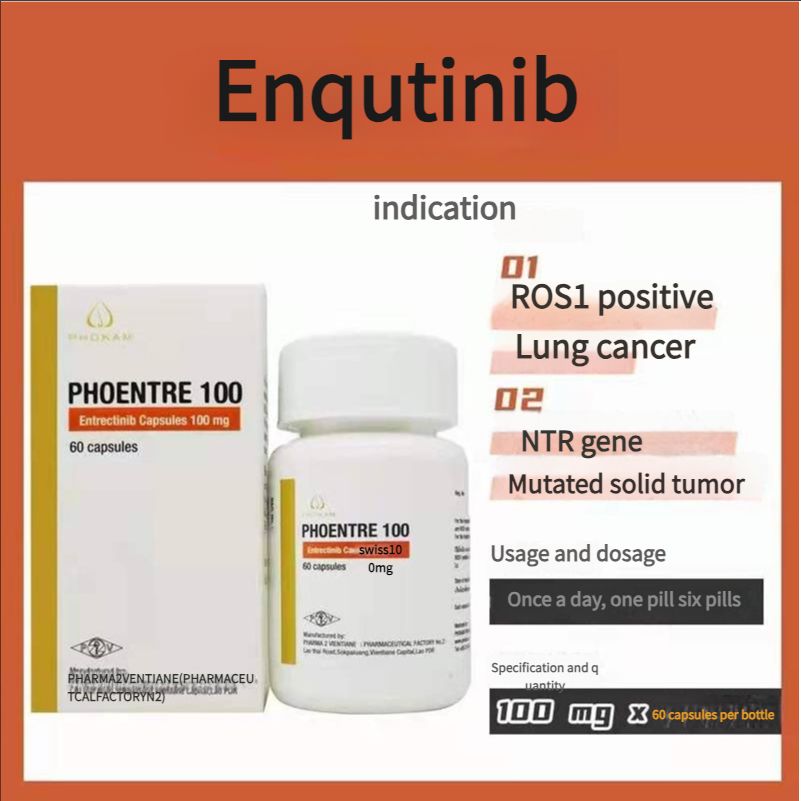
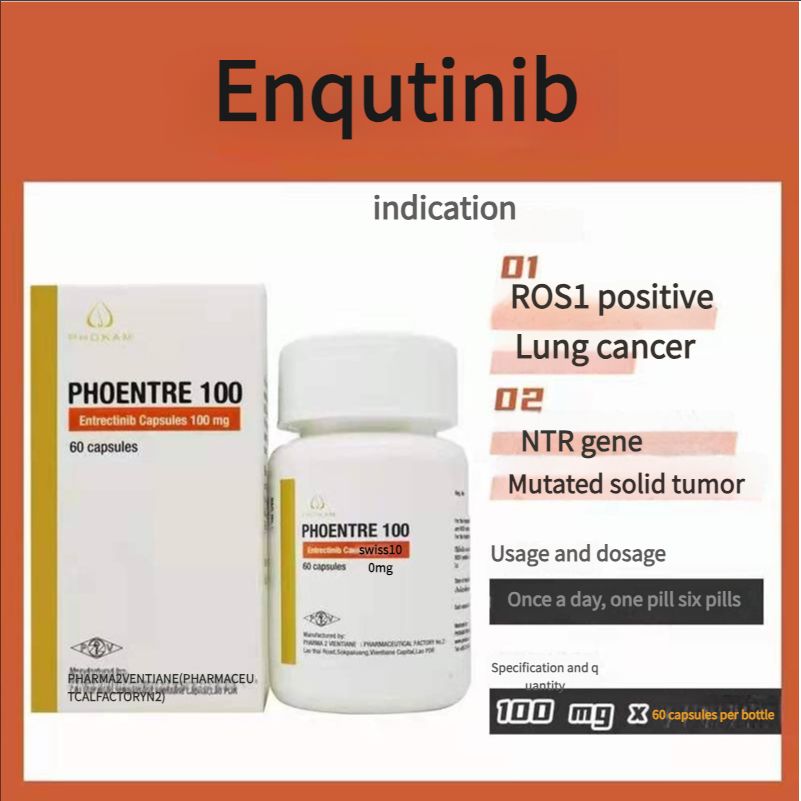
Non-small cell lung cancer, FDA approved, authoritative certification, Laos Second Pharmaceutical Factory Entrectinib 100mg 60 tablets
Indications:
100mg*60 tablets/box
1. Adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are ROS1-positive. 2. Adult and pediatric patients aged 12 years and above with solid tumors. The patient: ) has a neurotrophic tyrosine receptor kinase (NTRK) gene fusion without a known acquired resistance mutation: 2 metastatic or surgical resection may lead to severe complications; 3 has progressed after treatment or has no satisfactory alternative therapy.
Recommended dose: {For reference only, please follow the doctor's advice} 1. Recommended dose for ROS1-positive non-small cell lung cancer: 600 mg orally once daily; 2. Recommended dose for NTRK gene fusion-positive solid tumors: Adults: 600 mg orally once daily; Pediatric patients aged 12 years and above: The recommended dose is based on body surface area (BSA), as follows: BSA greater than 1.50m2, 600 mg orally once daily; BSA1.11 to 1.50m2: 500 mg orally once daily; BSA0.91 to 1.10 : 400 mg orally once daily.
Indications
ROS1-positive lung cancer
Solid tumors with NTRK gene mutations
Usage and dosage
Once a day, one to six tablets
Specifications
100 mg x 6O tablets 1 bottle