Just For You More
| id | 1921767778113073154 |
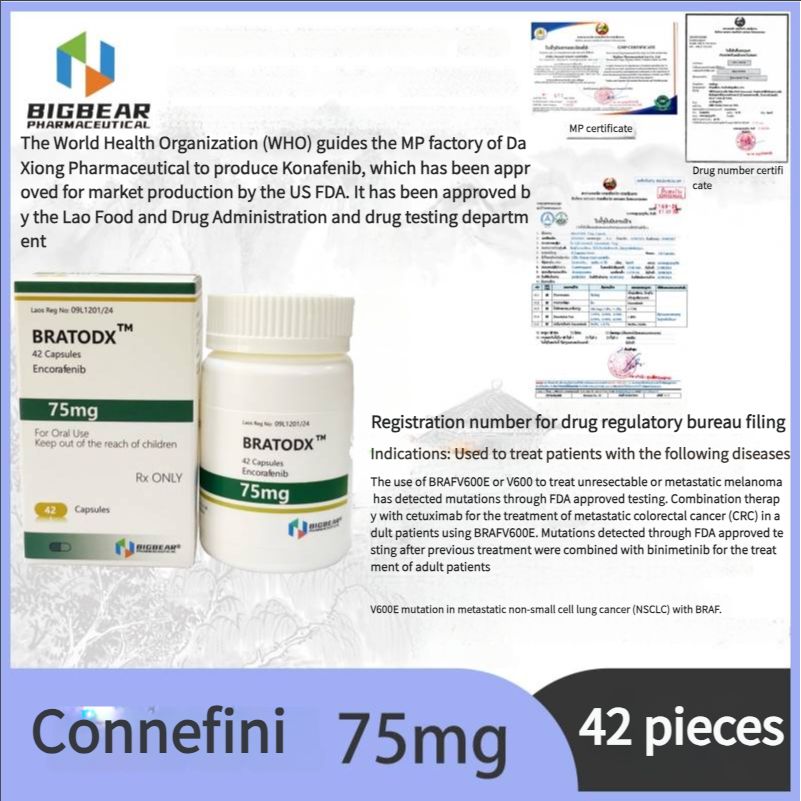
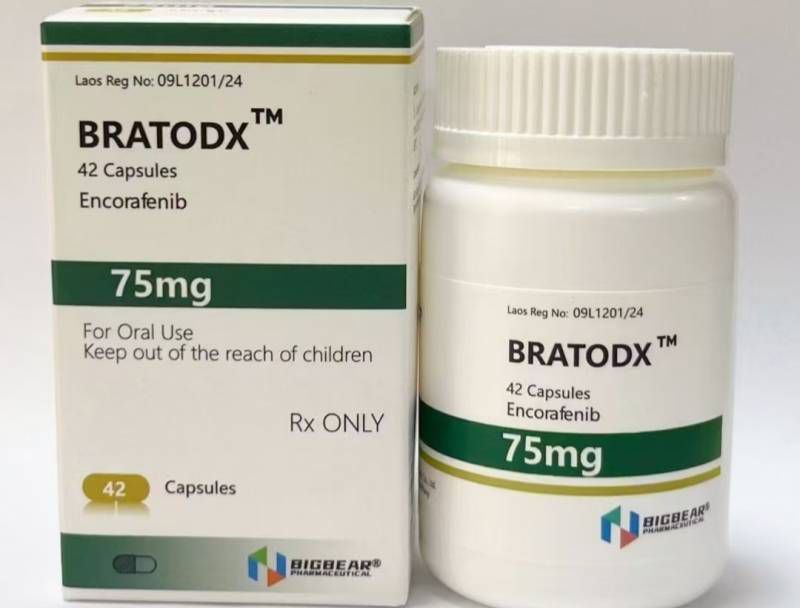
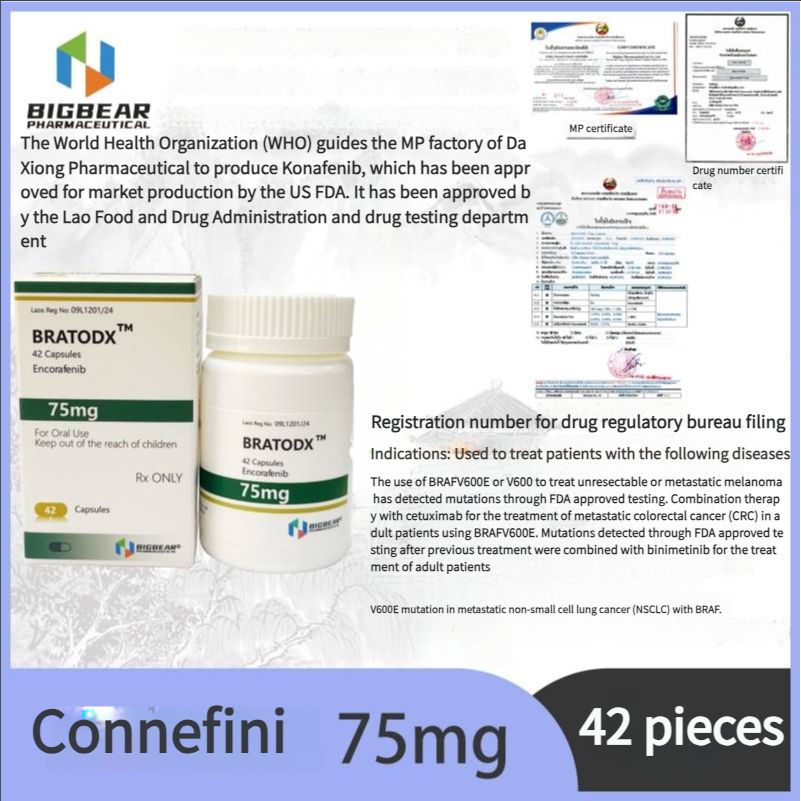
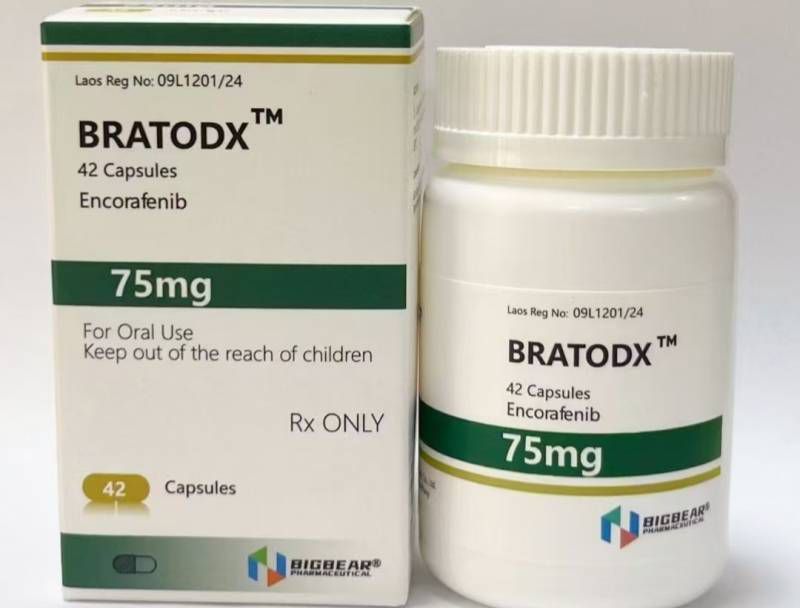
Melanoma, FDA approved, authoritative certification, Laos Daxiong Pharmaceutical Encofenib (Conaifenib) 75mg42 tablets/box
Indications: For the treatment of patients with unresectable or metastatic melanoma with BRAFV600E or V600K mutations detected by FDA-approved tests. In combination with cetuximab for the treatment of adult patients with BRAF V600E mutations detected by FDA-approved tests after prior treatment for metastatic colorectal cancer (CRC) In combination with binimetinib for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutations.
Indications:
75mg*42 tablets/box
Encofenib is used in combination with binimetinib to treat patients with unresectable or metastatic melanoma with BRAFV600E or V600K mutations detected by FDA-approved tests.
Recommended dose: {For reference only, follow doctor's advice}
Take 450 mg orally once daily, in combination with bimetinib. Can be taken with or without food.